Scientists from the Tokyo Institute of Technology, Tokyo Medical University, and Saitama Medical University have published findings that offer insights into how the drug pomalidomide benefits some patients with a cancer called multiple myeloma. Clinicians use pomalidomide to treat cases of multiple myeloma resistant to the more established drug lenalidomide, and the research team found that pomalidomide works by targeting a protein called ARID2. These findings may lead to new treatments for lenalidomide-resistant multiple myeloma.
Multiple myeloma is a cancer of the white blood cells, and patients diagnosed with this disease commonly die within 5 years. Clinicians often treat multiple myeloma with the drug thalidomide and structurally similar drugs, such as lenalidomide and pomalidomide. Pomalidomide offers therapeutic benefits in patients with lenalidomide-resistant multiple myeloma, but the reasons for its efficacy in such patients are sadly shrouded in mystery.
Now, a team of scientists from the Tokyo Institute of Technology, Tokyo Medical University, and Saitama Medical University led by Assistant Professor Junichi Yamamoto report novel findings concerning the therapeutic mechanisms of pomalidomide. The scientists show, in their paper published in Nature Chemical Biology, that pomalidomide causes the breakdown of a protein called ARID2. ARID2 promotes the “expression” of genes (the process by which the gene code is “read out” and used to create specific proteins) that are critical for the growth of multiple myeloma cells, so breaking down ARID2 is harmful to the cancer cells and beneficial to patients. “Our results provide new insights into the mechanisms of pomalidomide,” notes Prof. Yamamoto, “and we now have a better understanding of the clinical importance of ARID2 and related proteins in the context of multiple myeloma.”
To unravel how pomalidomide affects multiple myeloma cells, the scientists conducted a series of experiments involving cultured myeloma cells. Their experiments confirmed that treating the myeloma cells with pomalidomide reduced the levels of ARID2 within the cells, with greater pomalidomide concentrations and longer exposure times resulting in lower ARID2 levels. ARID2 promotes the expression of a gene called MYC, and the resulting MYC protein is very important for the growth of myeloma cells. Unsurprisingly, the cells that had lower ARID2 levels after being treated with pomalidomide also had reduced MYC levels. Figure 1 shows how treatment with pomalidomide for different lengths of time affected ARID2 and MYC levels within the myeloma cells.

Figure 1. Effects of pomalidomide treatment on ARID2 and MYC levels. Image credit: Tokyo Tech
In this figure, thicker bands for a protein indicate greater expression levels within myeloma cells. Treating the myeloma cells with pomalidomide reduced the expression levels for ARID2 and MYC, and longer exposure times caused greater reductions.
These findings offer a plausible explanation for why pomalidomide is effective at combating multiple myeloma. Interestingly, lenalidomide was not as effective as pomalidomide at reducing ARID2 levels and the expression of the MYC gene, and this may explain why some patients benefit from pomalidomide but not lenalidomide. Figure 2 provides a schematic overview of how pomalidomide and lenalidomide affected biochemical processes within the myeloma cells.
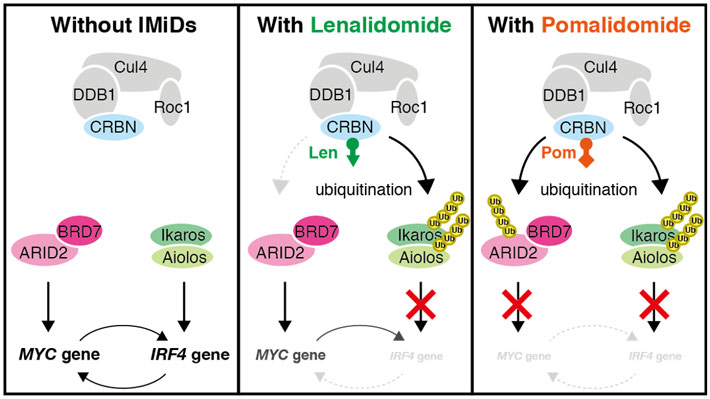
Figure 2. Effects of pomalidomide and lenalidomide on biochemical pathways inside the myeloma cells. Image credit: Tokyo Tech
Lenalidomide caused only a modest breakdown of the ARID2 protein and some reductions in expression of the MYC gene through another pathway, but pomalidomide had much more pronounced effects. “IMiDs” is short for “immunomodulatory drugs,” a category of drugs that includes both lenalidomide and pomalidomide.
The scientists also showed that ARID2 expression is associated with a poor prognosis and is higher in patients with relapsed or refractory multiple myeloma, most of whom acquire resistance to lenalidomide. Figure 3 shows the “survival probability” of multiple myeloma patients over time. The patient group with high ARID2 expression (dash line) indicated lower survival probability than the patient group with low ARID2 expression (solid line), suggesting that ARID2 is useful prognostic marker to predict overall survival periods of patients with multiple myeloma.
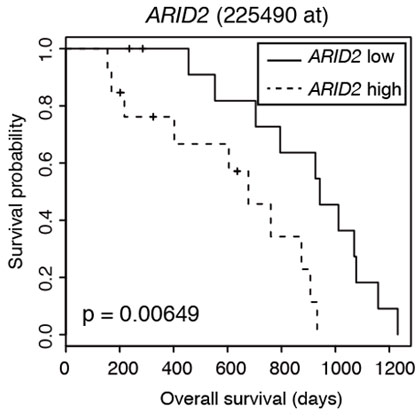
Figure 3. Survival analysis of multiple myeloma patients. Image credit: Tokyo Tech
This figure compares the survival probability of patients with low (solid line) and high (dash line) ARID2 expression over time. The high ARID2 expression group indicated unfavorable prognosis compared to the low ARID2 expression group.
Collectively, these results offer intriguing insights into how pomalidomide benefits patients with lenalidomide-resistant multiple myeloma, and these insights may help researchers develop new ways to treat this group of patients. “Our findings suggest that ARID2 is a promising target for overcoming lenalidomide resistance in patients with multiple myeloma,” notes Prof. Yamamoto. Armed with this knowledge, scientists can now find ways to improve survival times for patients diagnosed with this deadly form of cancer.
Reference:
J. Yamamoto, et al. “ARID2 is a pomalidomide-dependent CRL4CRBN substrate in multiple myeloma cells“. Nature Chemical Biology (2020)
Source: Tokyo Institute of Technology
