Brain tumors known as glioblastomas are among the most aggressive and malevolent forms of cancer. Median survival time without treatment is just nine months; with standard of care surgery and chemoradiation, it’s 15 to 16 months. Patients rarely survive more than a few years. In only a very small subset of glioblastoma patients, dubbed “exceptional responders,” do treatment results exceed expectations.
In a new study,published in the journal PNAS, researchers at University of California San Diego School of Medicine and University of Minnesota Medical School, suggest a novel explanation for glioblastoma aggression: Glioblastoma cells are able to reprogram key immune cells to promote tumor growth rather than fight it.
“These cancer cells appear to ‘brainwash’ our own immune cells and convert them from cells that fight cancer to cells that promote cancer,” said Judith Varner, PhD, professor in the departments of Pathology and Medicine at UC San Diego School of Medicine and co-senior author of the study. “Fortunately, we have figured out how glioblastoma cells subvert our immune system and can now reverse this cellular version of the ‘Stockholm syndrome.’”
Stockholm syndrome is a psychological response in which hostages or abuse victims develop an emotional bond with their captors or abusers.
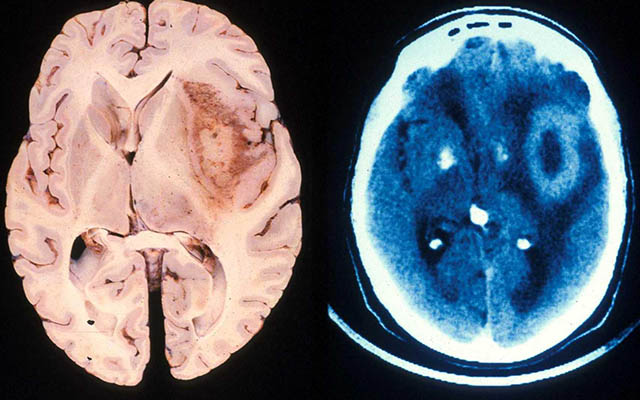
A glioblastoma tumor, as seen in actual brain tissue and MRI. Credit: UC San Diego
Researchers began their study by looking at gene expression profiles of glioblastoma specimens collected from 900 patients around the world, seeking to identify any novel commonalities among exceptional responders, who were defined as glioblastoma patients who survived more than two years after treatment.
Using a variety of advanced analytics, they discovered that one feature the tumor samples of exceptional responders shared was a surprising lack of microglia and macrophages — specialized immune cells that act as sentries and scavengers, identifying and removing abnormal cells, including cancer cells. As such, microglia and macrophages would be expected to congregate at tumor sites. In typical glioblastoma samples, they can make up more than half of the cells present.
“If microglia and macrophages normally fend off cancer cells, more of them should allow the body to better fend off the tumor,” said Jun Ma, MD, a researcher in the Department of Neurosurgery at the U of M Medical School and co-first author. “So we expected to see more of them in the exceptional responders. However, we found the contrary.”
Further investigation revealed that glioblastoma cells possess the ability to reprogram surrounding microglia and macrophages, negating their normal anticancer functions in favor of tumor growth promotion. In other words, the presence of numerous reconditioned immune cells in a glioblastoma tumor bode ill for the patient, while fewer — as seen in exceptional responders — indicated a better prognosis.
Varner said the key to reversing this cellular form of Stockholm syndrome and perhaps more effectively treating glioblastomas appears to lie with a protein called phosphoinositide-3-kinase gamma isoform or PI3Kγ. Activation of PI3Kγ converts microglia and macrophages from anticancer immune cells into hostage cells that promote cancer growth.
“In our animal glioblastoma models, treatment with drugs targeting (and inhibiting) PI3Kγ consistently resulted in impressively durable responses to chemotherapy,” said co-senior author Clark C. Chen, MD, PhD, head of the Department of Neurosurgery at U of M Medical School and a former faculty member at UC San Diego School of Medicine.
“We are eager to translate these findings into a human trial, with the hope of transforming every glioblastoma patient into an exceptional responder.”
Source: UC San Diego
