After you finish a milkshake or a bowl of guacamole, those dietary fats have a typical route through your body. New research from the laboratory of Dr. Natasza Kurpios, associate professor in the Department of Molecular Medicine in the Cornell College of Veterinary Medicine, has now revealed a key molecular mechanism that can send those fats on the wrong path, potentially resulting in debilitating diseases.
“Our research reveals a new mechanism in dietary fat absorption,” says Kurpios, “and highlights an alternative pathway for fats that could be explored in several disease states of the intestine.”

Intestinal villi of the mouse. Research from the Kurpios lab reveals the complex signaling that directs formation of intestinal lacteals, the lymphatic channels required for fat absorption in vertebrates. Image credit: Cornell University
Their study, published in the issue of the journal Cell Reports, looks closely at intestinal lacteals. Part of the lymphatic system, these tiny channels absorb dietary fat and direct those lipid molecules into the blood circulation. Scientists knew that lacteals were crucial for fat absorption, but how those lacteals form, and what causes their malfunction, remains unclear.
Kurpios and her team have studied how intestines first develop in embryos, and knew that the Pitx2 gene tells the embryo’s cells which way is left or right — a key mechanism in the gut’s twisting formation. They wanted to know if misregulation of Pitx2 might also be the culprit behind lacteal dysfunction.
They created genetically modified mice that made less of the Pitx2 protein, then bottle-fed the baby mice using fluorescent lipids, which allowed them to do state-of-the-art, three-dimensional fluorescent imaging to see where exactly those dietary lipids traveled in the mice’s bodies. “We found that these mice had intestinal lymphatics that didn’t function correctly,” says Kurpios. “Instead, the fats from their diet were shunted through a different route straight to the liver, where they accumulated and caused fatty liver disease.”
Looking deeper, Kurpios and her team showed that lacteals grow alongside, and depend on, neighboring vascular smooth muscle cells, known as 'star cells.' Functional gut lymphatics require support and signaling from these muscle star cells and depend on the Pitx2 left-right pathway to properly organize the gut lymphatics, protecting from fatty liver disease. This muscle-to-lymphatic signaling proceeds with messages encoded in the flow of reactive oxygen species between these cells, a novel and exciting feature of the study that will spawn further investigations in the intestine and elsewhere in the body.
Kurpios’ finding has real-world implications. For example, non-alcoholic fatty liver disease (NAFLD) is highly prevalent in the United States, with up to 25-33% of American adults estimated to be affected. The disease can lead to eventual loss of liver function. “Our study suggests that NAFLD can arise from altered intestinal lymphatic transport of fat – and not just from causes within the liver itself — inspiring discoveries of new mechanisms and regulators of this disease,” says Dr. Shing Hu, the lead author on the paper.
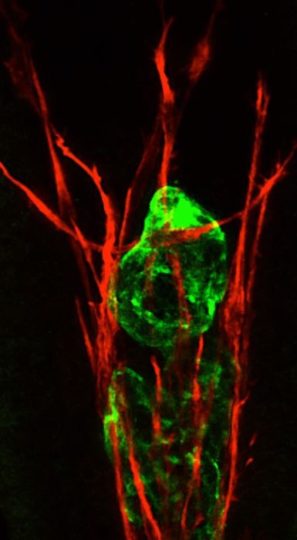
An intestinal lymphatic lacteal (green) requires the support of the surrounding vascular smooth muscle (red). The laterality gene Pitx2 is required for lymphatics and surrounding musculature to communicate, protecting from neonatal fatty liver disease. Image credit: Cornell University
The next step will be to develop a mouse model that allows more precise analyses of the ways in which Pitx2 works to direct the formation of gut lymphatics, and of the intestine as a complete organ. With funding from the Center for Vertebrate Genomics, the researchers will also collaborate with Dr. Iwijn De Vlaminck, associate professor at the Meinig School of Biomedical Engineering, to generate gene expression maps of all intestinal cell types at single-cell resolution and across key stages to remove Pitx2 with spatiotemporal precision.
“Collectively, we hope that our studies hold promise toward combating intestinal diseases and will prompt new discoveries on how fats are absorbed into the body,” Kurpios says.
Source: Cornell University
